Cardionerds: A Cardiology Podcast

303. CCC: Management of Ventricular Tachycardia and Electrical Storm in the CICU with Dr. Janice Chyou
CardioNerds Co-Founder, Dr. Amit Goyal, along with Series Co-Chairs, Dr. Yoav Karpenshif and Dr. Eunice Dugan, and episode Lead, Dr. Sean Dikdan, had the opportunity to expand their knowledge on the topic of ventricular tachycardia and electrical storm from esteemed faculty expert, Dr. Janice Chyou. Audio editing by CardioNerds Academy Intern, Dr. Maryam Barkhordarian.
Electrical storm (ES) is a life-threatening arrhythmia syndrome. It is characterized by frequently occurring bouts of unstable cardiac arrythmias. It typically occurs in patients with susceptible substrate, either myocardial scar or a genetic predisposition. The adrenergic input of the sympathetic nervous system can perpetuate arrythmia. In the acute setting, identifying reversible triggers, such as ischemia, electrolyte imbalances, and heart failure, is important. Treatment is complex and varies based on previous treatments received and the presence of intra-cardiac devices. Many options are available to treat ES, including medications, intubation and sedation, procedures and surgeries targeting the autonomic nervous system, and catheter ablation to modulate the myocardial substrate. A multidisciplinary team of cardiologists, intensivists, electrophysiologists, surgeons, and more are necessary to manage this complex disease.
The CardioNerds Cardiac Critical Care Series is a multi-institutional collaboration made possible by contributions of stellar fellow leads and expert faculty from several programs, led by series co-chairs, Dr. Mark Belkin, Dr. Eunice Dugan, Dr. Karan Desai, and Dr. Yoav Karpenshif.
Pearls • Notes • References • Production Team
CardioNerds Cardiac Critical Care Page
CardioNerds Episode Page
CardioNerds Academy
Cardionerds Healy Honor Roll
CardioNerds Journal Club
Subscribe to The Heartbeat Newsletter!
Check out CardioNerds SWAG!
Become a CardioNerds Patron!
Pearls and Quotes – Management of Ventricular Tachycardia and Electrical Storm
- Electrical storm is defined as 3 or more episodes of VF, sustained VT, or appropriate ICD shocks within 24 hours. It occurs more commonly in ischemic compared to non-ischemic cardiomyopathy, and it is associated with a poor prognosis and high cardiovascular mortality.
- The classic triad of electrical storm is a trigger, a myocardial susceptible substrate, and autonomic input perpetuating the storm.
- Triggers for electrical storm include ischemia, heart failure, electrolyte abnormalities, hypoxia, drug-related arrhythmogenicity, and thyrotoxicosis. A thorough evaluation of possible triggers is necessary for each patient, but it is uncommonly found. The evaluation may include laboratory studies, genetic testing, advanced imaging, or invasive testing.
- Acute treatment options involve acute resuscitation, pharmacotherapy with antiarrhythmics and beta-blockers, device interrogation and possible reprogramming, and sedation. Subacute treatment involves autonomic modulation and catheter ablation. Surgical treatments include sympathectomies and, ultimately, heart transplant.
- Catheter ablation is safe and effective for the treatment of electrical storm. In select patients, hemodynamic peri-procedural hemodynamic support should be considered.
Show notes – Management of Ventricular Tachycardia and Electrical Storm
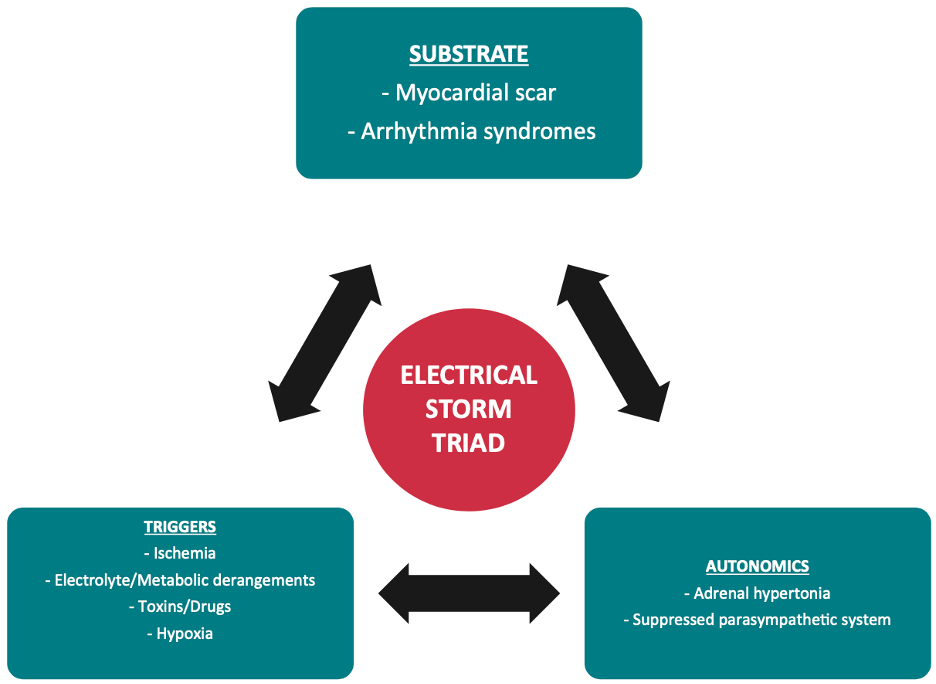
Simple diagram of the classic “triad” of ES (see reference 10).
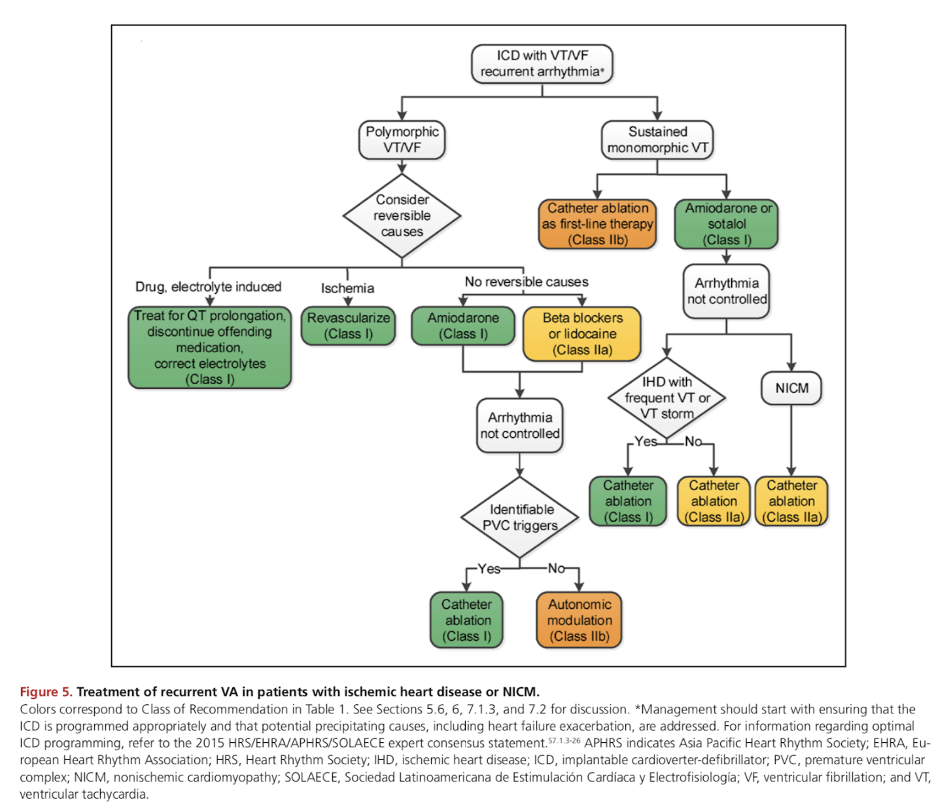
Treatment algorithm provided by the 2017 AHA/ACC/HRS guidelines (see reference 1).
1. Define electrical storm.
Electrical storm (ES), also called “arrhythmic storm” or “VT storm” refers to a state of cardiac instability associated with 3 or more episodes of VF, sustained VT, or appropriate ICD shocks within 24 hours. Sustained VT refers to 30 seconds of VT or hemodynamically unstable VT requiring termination in < 30 seconds. Incessant VT refers to continued, sustained hemodynamically stable VT that lasts longer than one hour. VT is incessant or recurrent when it recurs promptly despite repeated intervention for termination.1,2
In patients with ICDs for secondary prevention, ES is estimated to occur in 10-28% of patients.3–5 This incidence is much lower in patients who have ICDs implanted for primary prevention in whom the incidence has been estimated as low as 4% at 20 months of follow up.6 ES occurs at similar rates in patients with ischemic or non-ischemic cardiomyopathy.7
ES is associated with a poor prognosis and high cardiovascular mortality. The three-month mortality in patients with an episode of ES has been estimated at up to 18 times higher than in patients without any VT.6 Risk factors for the development of ES include male sex, advanced age, low left ventricular ejection fraction, use of class 1A antiarrhythmic drugs, and the presence of cardiovascular comorbidities.8,9
2. Evaluate the cause of VT storm (e.g., evaluation for ischemia, sarcoidosis , etc)
The classic triad of ES is a trigger, a substrate susceptible to ES, and autonomic input perpetuating the storm.10 Potential triggers are varied and typically include myocardial ischemia, decompensated heart failure, electrolyte abnormalities, hypoxia, drug-related arrhythmogenicity, and thyrotoxicosis.4,11 A clear trigger is often not found (only 13% of the time by some estimates).12 Searching for a trigger should not delay management decisions in the acute setting.
Structural heart disease unrelated to ischemia such as congenital heart disease and infiltrative cardiomyopathies can serve as the substrate for ES. Conditions related to genetic causes such as long QT syndrome or catecholaminergic polymorphic VT may be a rare etiology. These conditions represent an electrophysiologic substrate as opposed to a structural substrate.13
3. Choose an initial management strategy for patients with electrical storm in the CCU.
Treatment of ES is complex. The initial steps in management involve resuscitation, pharmacotherapy, device interrogation and reprogramming, and sedation. ACLS should be used in patients with pulseless VT or VF.
Patients with and without cardioverter-defibrillators may be treated differently. Defibrillations from an implanted device accentuate sympathetic tone and may perpetuate further arrhythmia.
Once a patient is stabilized, more advanced therapies involving autonomic modulation or catheter ablation (CA) can be utilized. In the patient with ischemia, emergent revascularization should be pursued. The need for mechanical circulatory support (MCS) should be determined. Inotropes and many vasopressors are sympathetic agonists and may worsen the arrhythmia by accentuating adrenergic tone, and so the benefits of improved hemodynamics need to be weighed against the risk of worsening electrical instability.
Initial pharmacotherapy in ES includes an antiarrhythmic drug and a beta blocker. Typically loading the patient with IV amiodarone and administering a non-selective beta blocker like propranolol is done. This combination has been shown in ES patients to have superior freedom of arrhythmia compared to using metoprolol.14 Propranolol’s superiority may also be due to its ability to cross the blood-brain barrier. Lidocaine has improved efficacy in ischemic VT.15,16 Procainamide has been shown to be useful in patients with hemodynamically stable VT.17
4. Identify predisposing conditions that should be managed to help treat electrical storm such as ischemia and AHF.
Identifying and managing specific triggers is an important initial step in the management of ES. Hypoxia on vital signs or evidence of decompensated HF on exam (with JVD, edema, crackles on auscultation) can implicate volume overload; this can be managed with diuresis.
Ischemic ECG changes on the 12-lead ECG when the patient’s ventricular arrhythmia is broken, can suggest myocardial ischemia. If ischemia is believed to be the trigger, urgent revascularization should be pursued while resuscitation is underway.
Blood work should include screening for electrolyte abnormalities and thyroid disease. Carefully screening the patient’s medication list and checking a digoxin level (when appropriate) can help detect drug-induced arrhythmia.
Once out of the acute setting, genetic testing may be important in patients without structural disease for determining an etiology. Idiopathic VT, Brugada syndrome, long QT syndrome, short QT syndrome, early repolarization syndrome, catecholaminergic polymorphic VT, arrhythmogenic right ventricular cardiomyopathy, and cardiac sarcoidosis are potential etiologies that may be related to ES.10
5. Recognize when to use general anesthesia to aid in the stabilization of electrical storm and incessant VT.
Intubation and deep sedation are immediate next steps to minimize the sympathetic drive contributing to the arrhythmia. This treatment is very effective at terminating arrythmia and preventing immediate recurrence.18,19 This step is used in the acute setting for ES that persists despite pharmacotherapy. Note that propofol is a negative inotrope with the potential to worsen heart failure in decompensated patients and precipitate shock.
In addition to breaking the sympathetic cycle that drives this pathophysiology, sedation mitigates some of the psychological stress that repeated ICD shocks can cause in patients.20
6. Describe considerations specific to patients with implanted ICDs.
If a patient with an ICD presents with ES, the device should be interrogated. It is important to confirm the shocks are appropriate. Inappropriate shocks can occur in up to 40% of patients with an ICD; causes may include atrial arrythmia, oversensing, and lead fracture.21,22 Inappropriate ICD shocks are associated with a worse outcome.
Overdrive pacing is a possible therapy to prevent ES. If the ES is hemodynamically stable, then the ICD therapies may be disabled manually or with the use of a magnet.
If anti-tachycardia pacing (ATP) treats the ventricular arrythmia effectively, adjusting these settings and increasing the use of ATP can mitigate unnecessary shocks in the future.23
7. Understand the role of catheter ablation in the management of electrical storm.
Research has shown an excellent response of ES to catheter ablation (CA). CA has a class 1 indication in patients with ES due to anti-arrhythmic drug refractory VA in both ischemic and nonischemic cardiomyopathy.2
Treatment of an initial episode of ES with CA has shown a reduction in all-cause mortality compared to other modalities.24 At nearly 1-2 years of follow up, nearly 90% of patients with ES that undergo CA are free from further ES, and roughly two-thirds of these patients are free from any ventricular arrythmia (VA) recurrence.25,26
CA is also relatively safe in this setting, with procedure-related mortality estimated to be less than 1%.27 Rapid transfer to an experienced catheter ablation capable facility is important in all critically ill patients with ES.
8. Consider when it may be appropriate to use mechanical support such as IABP, pVAD and ECMO.
Mechanical circulatory support (MCS) may be necessary to maintain adequate perfusion when the patient is suffering from cardiogenic shock due to unstable arrhythmia.
Patients with high risk for hemodynamic decompensation during CA can be preemptively supported with MCS. This practice has been shown to improve mortality compared to rescue or no MCS. 28,29 The PAAINESD score may be useful in identifying high risk patients. This score assigns numerical values to the following risk factors: pulmonary disease, age over 60 years, general anesthesia, ischemic cardiomyopathy, NYHA class III or IV, LV EF < 25%, VT storm, and diabetes mellitus. 2,28
An intra-aortic balloon pump may be sufficient but requires the patient to have enough adequate forward flow to generate a pulse. Extracorporeal membrane oxygenation (ECMO) has been studied and shows good long-term outcomes.29 Guidelines have a IIa recommendation for hemodynamic support with ECMO or a temporary LVAD during CA in select patients. 2
9. Discuss other strategies such as sympathectomies (stellate ganglion block vs. surgical), stereotactic radio ablation, and transplant for refractory cases.
There are several therapies available to treat ES that specifically target the autonomic nervous system (ANS).30 While sedation is used for this purpose acutely, other interventions seek to mitigate sympathetic activity in the subacute or chronic setting. These include stellate ganglion blockade (SGB), thoracic epidural anesthesia (TEA), cardiac sympathetic denervation (CSD), and renal artery sympathetic denervation (RSD).
Percutaneous SBG involves an injection of anesthetic directly into the stellate ganglia with or without ultrasound guidance. This is a temporizing measure that can be performed in the acute or subacute setting. It has shown complete suppression of VA in 50% of patients for the subsequent 48 hours.31
TEA involves the percutaneous administration of a local anesthetic directly into the thoracic epidural space. This is also a temporary treatment best used as a bridge to definitive treatment, such as CA or surgical denervation. In ES patients with a failed CA TEA can reduce VA up to 80% in most patients.32
CSD is a surgical measure that offers a more permanent solution. It can be useful in refractory ES that has not responded to multiple treatments. CSD has achieved 80% event-free survival up to 2 years.33 Guidelines recommend CSD in ES when beta-blockade, anti-arrhythmic drugs, and CA are deemed ineffective with a class IIb recommendation.1
RSD functions similarly but has the added benefit of being non-surgical and directly reducing catecholamine secretion.
Cardiac transplantation would be indicated in a patient that has unrelenting ES despite these aggressive measures. Patients with MCS and life-threatening arrhythmias qualify as status 1 for OHT.34 Whereas VT/VF without MCS by itself would qualify a patient as status 2.
References
- Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Circulation 2018;138(13):e272–391.
- Cronin EM, Bogun FM, Maury P, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace 2019;21(8):1143–4.
- Exner D v, Pinski SL, Wyse DG, et al. Electrical Storm Presages Nonsudden Death The Antiarrhythmics Versus Implantable Defibrillators (AVID) Trial. Circulation [Internet] 2001;103:2066–71. Available from: http://www.circulationaha.org
- Credner SC, Klingenheben T, Mauss O, Sticherling C, Hohnloser SH. Electrical Storm in Patients With Transvenous Implantable Cardioverter-Defibrillators Incidence, Management and Prognostic Implications. J Am Coll Cardiol 1998;32(7):1909–15.
- Bänsch D, Böcker D, Brunn J, Weber M, Breithardt G, Block M. Clusters of Ventricular Tachycardias Signify Impaired Survival in Patients With Idiopathic Dilated Cardiomyopathy and Implantable Cardioverter Defibrillators. J Am Coll Cardiol 2000;36(2):566–73.
- Sesselberg HW, Moss AJ, McNitt S, et al. Ventricular arrhythmia storms in postinfarction patients with implantable defibrillators for primary prevention indications: A MADIT-II substudy. Heart Rhythm 2007;4(11):1395–402.
- Streitner F, Kuschyk J, Dietrich C, et al. Comparison of ventricular tachyarrhythmia characteristics in patients with idiopathic dilated or ischemic cardiomyopathy and defibrillators implanted for primary prevention. Clin Cardiol 2011;34(10):604–9.
- Vergara P, Tung R, Vaseghi M, et al. Successful ventricular tachycardia ablation in patients with electrical storm reduces recurrences and improves survival. Heart Rhythm 2018;15(1):48–55.
- Emkanjoo Z, Alihasani N, Alizadeh A, et al. Electrical Storm in Patients with Implantable Cardioverter-Defibrillators Can It Be Forecast? Tex Heart Inst J 2009;36(6):563–7.
- Kowlgi GN, Cha YM. Management of ventricular electrical storm: A contemporary appraisal. Europace 2020;22(12):1768–80.
- Muser D, Liang J, Santangeli P. Electrical Storm in Patients with Implantable Cardioverter-defibrillators: A Practical Overview. J Innov Card Rhythm Manag 2017;8(10):2853–61.
- Stefan H. Hohnloser, Hussein R. Al-Khalidi, Craig M. Pratt, et al. Electrical storm in patients with an implantable defibrillator: incidence, features, and preventive therapy: insights from a randomized trial. Eur Heart J 2006;27(24):3027–32.
- Geraghty L, Santangeli P, Tedrow UB, Shivkumar K, Kumar S. Contemporary Management of Electrical Storm. Heart Lung Circ 2019;28(1):123–33.
- Chatzidou S, Kontogiannis C, Tsilimigras DI, et al. Propranolol Versus Metoprolol for Treatment of Electrical Storm in Patients With Implantable Cardioverter-Defibrillator. J Am Coll Cardiol 2018;71(17):1897–906.
- MacMahon S, Collins R, Peto R, Koster RW, Yusuf S, MacMahon M. Effects of Prophylactic Lidocaine in Suspected Acute Myocardial Infarction. J Am Med Assoc [Internet] 1988;260(13):1910–6. Available from: https://jamanetwork.com/
- Collinsworth KA, Kalman SM, Harrison DC. The Clinical Pharmacology of Lidocaine as an Antiarrhythymic Drug. Circulation [Internet] 1974;50(6):1217–30. Available from: http://ahajournals.org
- Ortiz M, Martin A, Arribas F, et al. Randomized comparison of intravenous procainamide vs. intravenous amiodarone for the acute treatment of tolerated wide QRS tachycardia: The PROCAMIO study. Eur Heart J 2017;38(17):1329–35.
- Martins RP, Urien JM, Barbarot N, et al. Effectiveness of Deep Sedation for Patients With Intractable Electrical Storm Refractory to Antiarrhythmic Drugs. Circulation 2020;142(16):1599–601.
- Bundgaard JS, Jacobsen PK, Grand J, et al. Deep sedation as temporary bridge to definitive treatment of ventricular arrhythmia storm. Eur Heart J Acute Cardiovasc Care 2020;9(6):657–64.
- Passman R, Subacius H, Ruo B, et al. Implantable Cardioverter Defibrillators and Quality of Life Results From the Defibrillators in Nonischemic Cardiomyopathy Treatment Evaluation Study. Journal of the American Medical Association Internal Medicine [Internet] 2007;167(20):2226–32. Available from: https://jamanetwork.com/
- Powell BD, Saxon LA, Boehmer JP, et al. Survival after shock therapy in implantable cardioverter-defibrillator and cardiac resynchronization therapy-defibrillator recipients according to rhythm shocked: The altitude survival by rhythm study. J Am Coll Cardiol 2013;62(18):1674–9.
- van Rees JB, Borleffs CJW, de Bie MK, et al. Inappropriate implantable cardioverter-defibrillator shocks: Incidence, predictors, and impact on mortality. J Am Coll Cardiol 2011;57(5):556–62.
- Wathen MS, DeGroot PJ, Sweeney MO, et al. Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators: Pacing fast ventricular tachycardia reduces shock therapies (PainFREE Rx II) trial results. Circulation 2004;110(17):2591–6.
- Morawski S, Pruszkowska P, Sredniawa B, Lenarczyk R, Kalarus Z. Long-term outcome of catheter ablation and other form of therapy for electrical storm in patients with implantable cardioverter-defibrillators. Journal of Interventional Cardiac Electrophysiology 2017;50(3):227–34.
- Carbucicchio C, Santamaria M, Trevisi N, et al. Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter-defibrillators : Short-and long-term outcomes in a prospective single-center study. Circulation 2008;117(4):462–9.
- Deneke T, Shin DI, Lawo T, et al. Catheter ablation of electrical storm in a collaborative hospital network. American Journal of Cardiology 2011;108(2):233–9.
- Nayyar S, Ganesan AN, Brooks AG, Sullivan T, Roberts-Thomson KC, Sanders P. Venturing into ventricular arrhythmia storm: A systematic review and meta-analysis. Eur Heart J 2013;34(8):560–9.
- Mariani S, Napp LC, lo Coco V, et al. Mechanical circulatory support for life-threatening arrhythmia: A systematic review. Int J Cardiol 2020;308:42–9.
- Baratto F, Pappalardo F, Oloriz T, et al. Extracorporeal Membrane Oxygenation for Hemodynamic Support of Ventricular Tachycardia Ablation. Circ Arrhythm Electrophysiol 2016;9(12).
- Zhu C, Hanna P, Rajendran PS, Shivkumar K. Neuromodulation for Ventricular Tachycardia and Atrial Fibrillation: A Clinical Scenario-Based Review. JACC Clin Electrophysiol 2019;5(8):881–96.
- Fudim M, Qadri YJ, Waldron NH, et al. Stellate Ganglion Blockade for the Treatment of Refractory Ventricular Arrhythmias. JACC Clin Electrophysiol 2020;6(5):562–71.
- Bourke T, Vaseghi M, Michowitz Y, et al. Neuraxial modulation for refractory ventricular arrhythmias: Value of thoracic epidural anesthesia and surgical left cardiac sympathetic denervation. Circulation 2010;121(21):2255–62.
- Li J, Liu Y, Yang F, et al. Video-Assisted Thoracoscopic Left Cardiac Sympathetic Denervation: A Reliable Minimally Invasive Approach for Congenital Long-QT Syndrome. Annals of Thoracic Surgery 2008;86(6):1955–8.
- Stevenson LW, Kormos RL, Young JB, Kirklin JK, Hunt SA. Major advantages and critical challenge for the proposed United States heart allocation system. Journal of Heart and Lung Transplantation 2016;35(5):547–9.






 Visit Podcast Website
Visit Podcast Website RSS Podcast Feed
RSS Podcast Feed Subscribe
Subscribe
 Add to MyCast
Add to MyCast