Cardionerds: A Cardiology Podcast

210. Family History of Premature ASCVD with Dr. Ann Marie Navar
CardioNerds (Amit Goyal and Daniel Ambinder), Dr. Ahmed Ghoneem (CardioNerds Academy Chief of House Taussig and medicine resident at Lahey Hospital), and Dr. Gurleen Kaur (Director of CardioNerds Internship and medicine resident at Brigham and Women’s Hospital) discuss family history of premature ASCVD with Dr. Ann Marie Navar, Preventive Cardiologist and Associate Professor in the Departments of Internal Medicine and Population and Data Sciences at UT Southwestern Medical Center. They discuss the art of soliciting a nuanced family history, refining cardiovascular risk using risk models and novel markers, counseling patients with elevated risk, and more. Show notes were drafted by Dr. Ahmed Ghoneem and reviewed by Dr. Gurleen Kaur. Audio editing was performed by CardioNerds Intern, student Dr. Adriana Mares.
For related teaching, check out this Tweetorial about CAC by Dr. Gurleen Kaur, the Family History of Premature ASCVD Infographic by Dr. Ahmed Ghoneem, and the CardioNerds Cardiovascular Prevention Series.
CardioNerds Cardiovascular Prevention Page
CardioNerds Episode Page

Show notes – Family History of Premature ASCVD with Dr. Ann Marie Navar
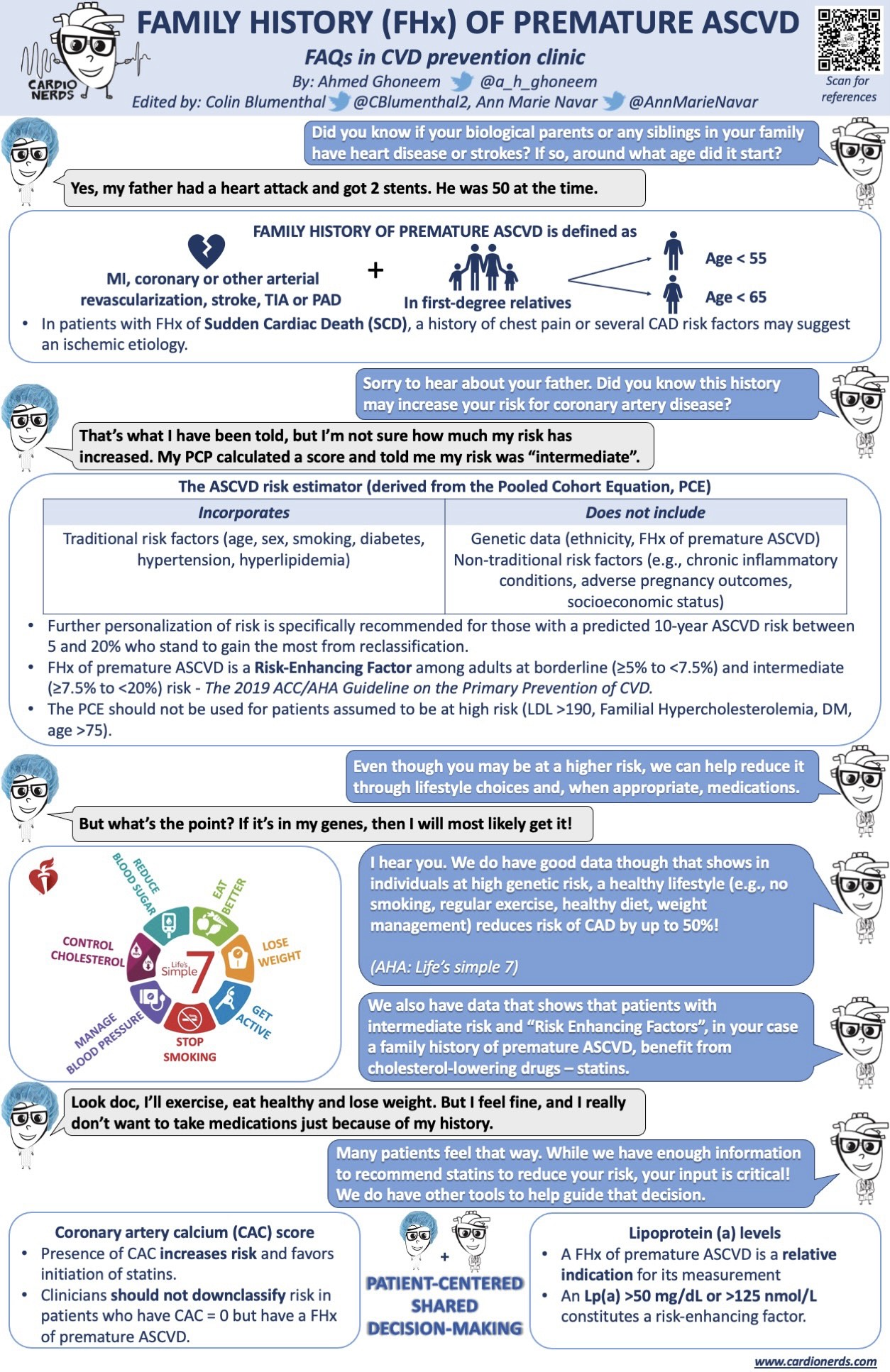
Patient summary: Mr. B is a 51-year-old gentleman who is referred to CardioNerds Prevention Clinic by his PCP. He does not have a significant past medical history. He is a former smoker but quit 2 years ago. His BP in clinic today is 138/84; he is not on any antihypertensives. His most recent lipid profile 2 weeks prior showed a total cholesterol level of 250 mg/dL, a TG level of 230 mg/dL, an LDL cholesterol of 174 mg/dL, and an HDL cholesterol of 30 mg/dL. He tells us that his father had a “heart attack” at the age of 52, and he would like to further understand his own risk. We calculate his ASCVD risk score, and it is 9.8%.
1. What constitutes a positive family history (FHx) of premature ASCVD? What is an approach to the art of soliciting the FHx from our patients?
- Definition of family history of premature ASCVD: the history of an atherosclerotic event (e.g., myocardial infarction or stroke) in a male first degree relative before the age of 55 or a female first degree relative before the age of 65.
- Dr. Navar’s approach to soliciting a family history:
- Lead with a general question such as “what do you know about any medical conditions that run in your family?”.
- Then ask more specific questions about the parents and siblings, such as “Is your mother still alive? How long did she live? Has she ever had a heart attack or stroke?”
- If the answer is yes, ask about how old they were at the time of the event.
- A challenging aspect of the FHx can be eliciting the difference between atherosclerotic events and sudden cardiac death. While atherosclerotic diseases are a much more common cause of unexplained sudden death, it’s important that we don’t miss the opportunity to identify inherited cardiomyopathies, channelopathies, inherited aortopathies or other heritable SCD syndromes.
- Lead with a general question such as “what do you know about any medical conditions that run in your family?”.
2. Is the “dose” of family history important (for example: the number of affected relatives, the closeness of those relationships, the age of onset)?
- While conducting studies to test this may be difficult, the few studies that have looked at the number of affected relatives have found a dose-response type relationship, where increasing number of relatives affected increases the risk of heart disease.1,2
3. How does a family history affect cardiovascular risk stratification?
FHx of premature ASCVD does not improve the predictive ability of the Pooled Cohort Equations (PCE) at a population level. Therefore, it does not factor into the ASCVD risk calculation utilizing the PCE.
However, it enhances the patient’s risk at an individual level. The ACC/AHA guidelines recognize FHx of premature ASCVD as a risk-enhancing factor [together with CKD, chronic inflammatory conditions such as psoriasis, primary hypercholesterolemia, high-risk ethnicity such as South Asian ancestry, metabolic syndrome, history of premature menopause (before age 40 y) and history of pregnancy-associated conditions that increase later ASCVD risk, such as preeclampsia].3
Dr. Navar’s advice is to think of the PCE as a starting point during risk assessment, followed by searching for the other risk-enhancing factors, to come up with a more tailored risk assessment for that individual patient.
4. After we explained to our patient the enhanced risk, he tells us that he feels like his fate is sealed and that nothing he can do would improve his outcomes because it’s in his genes. We have data though, that shows that healthy lifestyles reduce the risk of coronary artery disease and individuals at high genetic risk of CAD.4
How would you handle this challenging conversation and counsel our patient?
- We should always think about how risk conversations can affect our patients emotionally. Many patients come to CV prevention clinic because they want to know what they can do about their risk. A “scared straight” approach can be de-motivating for these patients.
- “It’s never too late to start cardiovascular prevention. And there are never patients that are too high risk that we can’t do anything to lower their risk of heart disease” can be a reassuring beginning to the conversation.
- Reinforce any work that the patient has already done to lower their risk of heart disease such as quitting smoking. This shows the patients that they have already started making a difference.
- This can then be followed by discussing the available tools to lower this patient’s risk: diet modification, exercise, weight management, control of diabetes, control of blood pressure, statins, etc.
5. In addition to lifestyle management, would you recommend a statin for Mr. B, who has an intermediate CVD risk and risk enhancing factors such as positive family history?
- Dr. Navar’s short answer is YES. Even though the patient is at intermediate risk, his actual risk is higher given his family history. “The cornerstone of long-term risk prevention for this patient is going to be a statin”.
- Dr. Navar usually approaches this conversation using a simple yet effective analogy: “imagine that you’re out shopping, and there’s a vitamin that will lower your risk of heart disease over your lifetime by 30 to 50%, depending on your cholesterol level and how much your cholesterol is lowered. It’s very safe. It has minimal side effects and it’s all of $3 a month. But unlike vitamins, we actually have randomized control trial data that show that they make a difference. Would you take it?”.
- It’s important to identify the LDL goal for each patient before prescribing a statin.
6. Our patient is still uncertain about starting a statin mainly because he’s asymptomatic and doesn’t feel that his family history is a strong enough reason for him to have to take a daily medication for the rest of his life. The ACC/AHA guidelines highlight the importance of shared decision making with our patients. They also recommend measuring the coronary artery calcium or CAC score in intermediate risk patients to help facilitate a more informed risk discussion. Would a CAC score be of benefit in our patient’s case?
- The initial step in this decision-making process is to identify what are the patient’s thoughts on taking statins, i.e where they are on the “Statin Spectrum” as Dr. Navar eloquently described. It is important to discuss the current evidence for the benefit of statins, and that the relative risk reduction we see per degree of LDL lowering is actually highest in the youngest and lowest risk groups.
- Some patients are engaged and interested in taking statins from the beginning. It may not be beneficial to measure CAC scores in these patients.
- Other patients, like our patient, remain uncertain about statins. These patients are great candidates for CAC score, which acts as a “tie-breaker” in this situation. Being aware of the presence of CAC in their arteries can be a strong motivator for these patients to start statin therapy.
- In non-smoking patients with CAC=0, it would be reasonable to hold statin therapy and repeat a CAC score in five years (assuming no major change in other risk factors such as diabetes, return to smoking, etc.).5
- It is important to recognize the caveats of CAC scores:
- The ACC/AHA guidelines identify some patients whom clinicians should not de-risk despite a CAC=0, such as active smokers.
- CAC is less prevalent in young people, and less prevalent in women compared to men, raising the possibility of false reassurance.
- CAC testing involves a small, but not insignificant, amount of radiation exposure.
- It is often not covered by insurance.
- The ACC/AHA guidelines identify some patients whom clinicians should not de-risk despite a CAC=0, such as active smokers.
7. There’ve been a lot of interest in novel lipid biomarkers such as lipoprotein(a) and Apolipoprotein B, and how elevated blood levels of these markers may be associated with ASCVD risk and are recognized as risk enhancing factors. How do you incorporate testing for Lp(a) and ApoB into your practice?
- Lipoprotein (a)
- It is a type of cholesterol particle that looks like an LDL particle, but it has an additional protein on it on the surface. It doesn’t correlate with LDL levels or any of the traditional risk factors for ASCVD, and Lp(a) levels are very consistent throughout lifetime. Moreover, it is genetically determined, and elevated Lp(a) levels usually run in families.
- The guidelines recommend measuring Lp(a) in patients with family history of premature ASCVD.3
- There are no commercially available treatments at this time that lower Lp(a), however a number of novel therapies are currently in clinical trials.
- Despite that fact, several preventive cardiologists (such as Dr. Navar) may measure Lp(a) to identify patients at increased risk, and subsequently target lower LDL levels in these patients (LDL<70, and in patients with multiple risk factors or established CVD, LDL<55).
- It is a type of cholesterol particle that looks like an LDL particle, but it has an additional protein on it on the surface. It doesn’t correlate with LDL levels or any of the traditional risk factors for ASCVD, and Lp(a) levels are very consistent throughout lifetime. Moreover, it is genetically determined, and elevated Lp(a) levels usually run in families.
- Apolipoprotein B
- It reflects the total number of atherogenic lipoprotein particles, in contrast to LDL-C which measures the volume of LDL (but not the number of LDL particles).6
- ApoB and LDL-C are not always concordant. A discordant phenotype exists, in which patients have a low LDL-C but a high ApoB; this confers an increased ASCVD risk.
- Measuring ApoB is easy, cheap and is not affected by triglycerides so can be measured in a non-fasting state.
- Check out this Tweetorial about ApoB by Dr. Christian Faaborg-Andersen.
- It reflects the total number of atherogenic lipoprotein particles, in contrast to LDL-C which measures the volume of LDL (but not the number of LDL particles).6
8. Our patient decided to proceed with a coronary CT to calculate his CAC and he’ll consider taking a statin based on the results. However, he asks one final question. He has a 20-year-old son and a 25-year-old daughter. Should they be screened for ASCVD?
- Remember, it’s never too early to start thinking about cardiovascular prevention.
- It is important for this patient’s children to understand that they have a family history of ASCVD, that they could be at an increased risk, and that they should start adopting a healthy lifestyle early on.
- Screening can help identify individuals who may be eligible for early intervention, such as those with Familial Hypercholesterolemia (FH). Early identification and management of people with FH can vastly improve their outcomes.
References
1. Silberberg JS, Wlodarczyk J, Fryer J, Robertson R, Hensley MJ. Risk associated with various definitions of family history of coronary heart disease. The Newcastle Family History Study II. Am J Epidemiol. 1998;147(12):1133-1139. https://pubmed.ncbi.nlm.nih.gov/9645791/
2. Scheuner MT, Whitworth WC, McGruder H, Yoon PW, Khoury MJ. Expanding the definition of a positive family history for early-onset coronary heart disease. Genet Med. 2006;8(8):491-501. https://pubmed.ncbi.nlm.nih.gov/16912580/
3. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. https://www.ahajournals.org/doi/10.1161/CIR.0000000000000678
4. Khera AV, Emdin CA, Drake I, et al. Genetic Risk, Adherence to a Healthy Lifestyle, and Coronary Disease. New England Journal of Medicine. 2016;375(24):2349-2358. https://www.nejm.org/doi/full/10.1056/nejmoa1605086
5. Dzaye O, Dardari ZA, Cainzos-Achirica M, et al. Warranty Period of a Calcium Score of Zero: Comprehensive Analysis From MESA. JACC Cardiovascular imaging. 2021;14(5):990-1002. https://pubmed.ncbi.nlm.nih.gov/33129734/
6. Sniderman AD, Thanassoulis G, Glavinovic T, et al. Apolipoprotein B Particles and Cardiovascular Disease A Narrative Review. JAMA Cardiology. 2019; 4(12):1287-1295. https://pubmed.ncbi.nlm.nih.gov/31642874/






 Visit Podcast Website
Visit Podcast Website RSS Podcast Feed
RSS Podcast Feed Subscribe
Subscribe
 Add to MyCast
Add to MyCast