Cardionerds: A Cardiology Podcast

319. Case Report: Caring for the Middle Child of Pulmonary Embolism – Texas Heart Institute
CardioNerds cofounders Dr. Amit Goyal and Dr. Daniel Ambinder join Dr. Isabel Balachandran, Dr. Diego Celli from the Texas Heart Institute. They discuss the nuances of risk stratification management of intermediate risk pulmonary embolism. The ECPR for this episode was provided by Dr. Alam Mahboob (Associate Professor of Medicine at Baylor College of Medicine and the Department of Medicine and Associate Program Director for the Cardiovascular Disease Fellowship Program at Baylor). Audio editing by CardioNerds Academy Intern, Dr. Chelsea Amo Tweneboah.
“To study the phenomena of disease without books is to sail an uncharted sea, while to study books without patients is not to go to sea at all.” – Sir William Osler. CardioNerds thank the patients and their loved ones whose stories teach us the Art of Medicine and support our Mission to Democratize Cardiovascular Medicine.
US Cardiology Review is now the official journal of CardioNerds! Submit your manuscript here.

CardioNerds Case Reports Page
CardioNerds Episode Page
CardioNerds Academy
Cardionerds Healy Honor Roll
CardioNerds Journal Club
Subscribe to The Heartbeat Newsletter!
Check out CardioNerds SWAG!
Become a CardioNerds Patron!
Pearls – Caring for the Middle Child of Pulmonary Embolism – Texas Heart Institute
- Submassive pulmonary embolism is defined as an intermediate risk group of acute pulmonary embolism, which presents with signs of RV dysfunction and myocardial injury without hemodynamic instability.
- The AHA, ACCP, and ESC have variable definitions of submassive PE. Non-invasive tools such as EKG, TTE, and CT are critical to defining RV dysfunction. The Pulmonary Embolism Severity Index (PESI) score is a validated tool to help risk stratify patients with PE.
- Advanced therapies for submassive PE include systemic thrombolysis, catheter-based intervention, surgical embolectomy, and mechanical circulatory support. The decision between these therapies is based on individual patient risk profiles, local expertise, and the risk of major bleeding.
- There is a spectrum of long-term complications after an acute PE, ranging from post PE syndrome to CTEPH (chronic thromboembolic pulmonary hypertension) caused by a maladaptive vascular remodeling from residual thrombus or arteriopathy. Thrombolytic therapies are still controversial in reducing the risk of post PE complications.
- PERT is a multidisciplinary group of clinicians who can rapidly assess and triage patients with acute PE, coordinate access to medical and advanced therapies, and provide the necessary follow up care.
Show Notes – Caring for the Middle Child of Pulmonary Embolism – Texas Heart Institute
How do you define “submassive” pulmonary embolism?
- Venous thromboembolism, which includes deep vein thrombosis and acute pulmonary emboli (PE) are the third most common cardiovascular disorder in the United States with approximately 900,000 cases occurring each year (1). The morbidity and mortality associated with pulmonary emboli are also great, with approximately 33% of PE cases being fatal (1).
- Until recently, PE was previously classified into massive or non-massive. Massive PE was defined as those with cardiogenic shock. A newer group, “submassive PE”, was defined as an “intermediate” risk group. According to the American Heart Association (AHA) Scientific Statement on the management of massive and submassive PE, patients in this group presented with signs of RV dysfunction and myocardial necrosis without hemodynamic instability (2).
- Intermediate-risk PE covers a broad range of risk and management decisions remain challenging. Intermediate-risk PE convers increased risk for mortality and complications compared with low-risk PE.
- Until recently, PE was previously classified into massive or non-massive. Massive PE was defined as those with cardiogenic shock. A newer group, “submassive PE”, was defined as an “intermediate” risk group. According to the American Heart Association (AHA) Scientific Statement on the management of massive and submassive PE, patients in this group presented with signs of RV dysfunction and myocardial necrosis without hemodynamic instability (2).
How do you risk-stratify intermediate-risk pulmonary emboli?
- The AHA, American College of Chest Physicians (ACCP), and European Society of Cardiology (ESC) have variable definitions of submassive PE and which biomarkers should be used (1,3). The contents are summarized as below (Table 1)
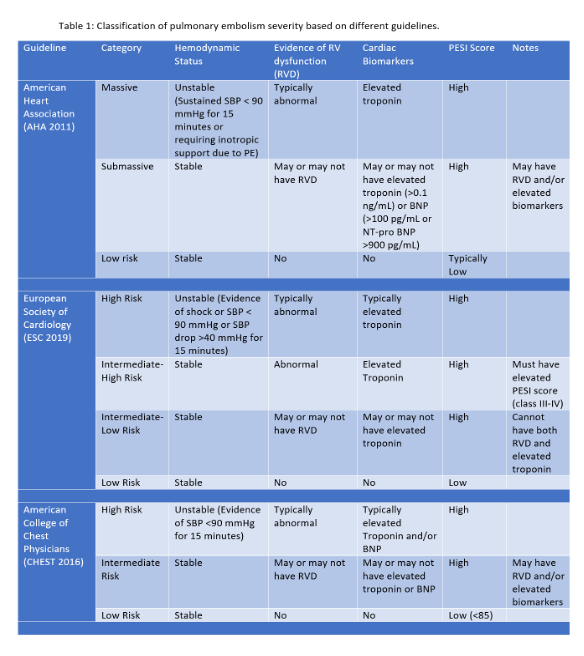
- Each major guideline highlights the importance of the evaluation of RV dysfunction (RVD) and elevated biomarkers. To summarize, the AHA defines submassive PE with either RVD or elevated biomarkers, specifically troponin levels (2). The ACCP similarly defines an intermediate risk PE with either RVD or elevated biomarkers, though with both elevated BNP/NT-proBNP or troponin levels (4). Finally, the ESC subdivides intermediate risk into intermediate-high and intermediate-low risk groups based on the PESI score and if there are elevated troponin levels (3).
- As of 2019, the AHA published a consensus statement revising the nomenclature of PE. The terminology is now high risk, intermediate risk, and low risk (4).
- The AHA 2011 guidelines define RVD based on the following non-invasive tools (EKG, CT and transthoracic echocardiography) (2).By EKG, concerning changes include a new or incomplete right bundle branch block, anteroseptal ST elevation or depression, or anteroseptal T wave inversions. Other pertinent findings include sinus tachycardia (the most common abnormality), atrial arrhythmias, low voltages, right axis deviation, S1Q3T3, or Qr pattern in V1 (2).By CT, RV enlargement is defined as an RV to LV diameter ratio > 0.90 (2).By TTE, RV enlargement (RV to LV diameter ratio > 0.9), systolic dysfunction (TAPSE <17 mm or S’ <0.1 m/s) or hypokinesis (sometimes with the preservation of apical contractility- aka McConnell’s sign) (2). There can also be signs of pulmonary hypertension, which include flattening of the interventricular septum during systole or a tricuspid regurgitant velocity > 2.7 m /sec. Finally, there can also be direct visualization of thrombus within the right sided chambers (5).
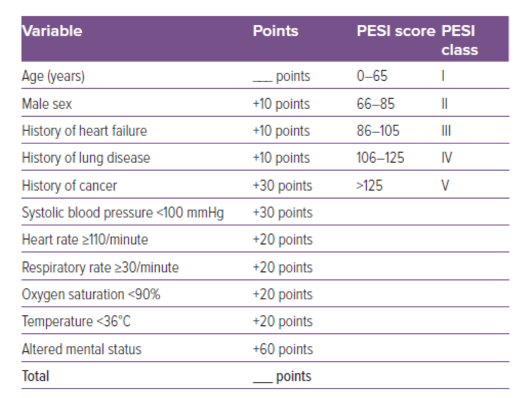
- The PESI or Pulmonary Embolism Severity Index is a validated tool using markers such as age, sex, vital signs, presence of hypoxemia, altered mental status, and comorbidities (such as cancer, heart failure, and chronic lung disease) to risk stratify patients with PE. A high PESI score is suggestive of an elevated 30-day mortality (6).
What is the general approach to therapeutic interventions and treatment for submassive PE?
- With confirmed PE and no contraindications to systemic anticoagulation prompt use of low molecular weight heparin (LMWH), unfractionated heparin, or fondaparinux should be used. For those confirmed with heparin induced thrombocytopenia, a non-heparin based anticoagulant (such as argatroban or bivalirudin) should be used (2). In regards to direct oral anticoagulation (DOAC), in large studies (including the EINSTEIN and AMPLIFY trials), patients with submassive PE predominantly received LMWH prior to DOAC initiation. It is uncertain whether direct initiation of DOAC is comparable in outcomes (1).
- Treatment with systemic anticoagulation alone in normotensive patients with RV dysfunction, is controversial and the use of more aggressive therapies has been studied extensively. Advanced therapies for submassive PE include systemic thrombolysis, catheter-based interventions, surgical embolectomy, and mechanical circulatory support. The decision between these therapies is based on individual patient risk as well as the risk of major bleeding, best guided by pulmonary embolism response teams (PERT) (7).
- Finally, surgical embolectomy is considered in those with submassive and massive PE in whom fibrinolytic therapy has failed or is contraindicated. Other indications include paradoxical emboli, clot in transit, or hemodynamic collapse. In large, high-volume centers, this has been found to be a safe and effective approach (1).
- Hemodynamic support is important to consider for management of RV failure. If a limited intravenous fluid trial fails, early vasopressor and inotropic support should be initiated. ECMO is indicated for hemodynamic and ventilatory support in patients with severe RV failure and refractory cardiogenic shock (7).
When do you consider systemic thrombolysis or catheter directed therapies for patients with intermediate-risk PE?
- Systemic thrombolysis works by rapidly acting on acute thrombus, thereby reducing pulmonary pressures and RV dysfunction, along with improving hemodynamics. This has been shown to be superior to anticoagulation alone in massive PE with reductions in mortality at the cost of increased major bleeding (1,2). The PEITHO trial was the largest randomized control trial of systemic thrombolysis in PE. Thrombolytic treatment with Tenecteplase reduced a composite outcome of all-cause mortality at 7 days and hemodynamic decompensation versus anticoagulation with heparin alone. This, however, came at the expense of an increased risk of major bleeding (including intracranial hemorrhage) (8). Given this concern, the MOPPET-3 trial in 2013 evaluated the effect of low dose thrombolysis on outcomes in patients with submassive PE. It was found that low dose tPA reduced the incidence of pulmonary hypertension. However, tPA did not reduce the rates of a combined outcome of recurrent PE or all-cause mortality (9).
- Catheter-based therapy, including pharmacomechanical therapy, catheter-directed thrombolysis, and mechanical embolectomy, is a widely studied approach for treating pulmonary embolism (PE) (10). In 2013, the ULTIMA trial compared CDT plus anticoagulation versus anticoagulation alone in intermediate-high risk PE and found that CDT resulted in a statistically significant improvement in the right ventricular/left ventricular (RV/LV) ratio at 24 hours. At 90 days, there was no difference in mortality or major bleeding events between the two groups (11). A recent meta-analysis compared CDT to systemic anticoagulation (sAC) alone and showed lower rates of in-hospital, 30-day, and 90-day mortality, with no differences in major or minor bleeding or blood transfusions (10).
- The most widely studied technique is ultrasound-facilitated catheter-directed fibrinolysis (EKOS), which combines local fibrinolysis with mechanical thrombectomy. The SEATTLE II trial found a reduction in mean RV/LV ratio and mean pulmonary artery systolic pressure at 48 hours post-thrombolysis. Although major bleeding occurred in 10% of patients, there was no intracranial hemorrhage (12). The OPTALYSE trial aimed to determine the optimal dose of tissue plasminogen activator (TPA) and found that a lower dose delivered over a shorter duration resulted in improved right ventricular function and reduced clot burden compared to baseline (13). In contrast, the SUNSET trial failed to demonstrate better outcomes or safety profile between CDT and systemic anticoagulation (14).
- Pure mechanical catheter thrombectomy, such as the FlowTriever system, can be used in patients with contraindications to fibrinolysis and has been shown to result in a 25% reduction in RV/LV ratio in submassive PE in a multicenter study. However, there is still a lack of significant mortality data or large randomized controlled trials for catheter-based therapy (15).
What are common complications after submassive pulmonary emboli?
- There is a spectrum of long-term complications after an acute PE (including intermediate risk PE), called Post-PE syndrome. These include limitations in functional capacity, cardiopulmonary dysfunction, and an overall decreased quality of life. In addition, chronic thromboembolic pulmonary disease (CTEPH) can occur which is characterized by persistent pulmonary vasoconstriction and arterial obstruction (16). The pathophysiology behind these complications are likely due to maladaptive vascular remodeling from residual thrombus or arteriopathy which results in increased pulmonary vascular resistance and possibly further RV dysfunction (17).
- In the ELOPE study, a prospective cohort study, almost half of acute PE patients had exercise limitations at 1 year on cardiopulmonary exercise testing, based on a VO2-max <80% predicted (18). Further, another study found that approximately 45% to 52% of surviving PE patients exhibit a New York Heart Association Score of ≥2 even up to 3 years after the acute incident (19).
- The evidence for thrombolytics is still controversial with regards to impact on long term outcomes. Though the PEITHO study has the largest long term follow up of advanced therapies and thrombolysis, there was no significant reduction in chronic PE complications, RV dysfunction, or persistent symptoms with thrombolysis (1,2). However, several smaller studies have now reported long term benefits of thrombolysis (TOPCOAT and MOPPET trial) with improved functional outcomes and quality of life (2).
What is a PERT team and what is their role in the management of pulmonary emboli?
- A PERT or pulmonary embolism response team brings together a multidisciplinary group of clinicians who can rapidly assess and provide treatments for acute PE, who can exercise a full range of medical, endovascular, and surgical therapies, and who can provide appropriate follow up for patients (2, 17). PERT teams’ structures are variable based on the institution and the optimal PERT structure is not fully known. Often, however, a team will include emergency medicine, critical care, non-invasive and interventional cardiology, vascular medicine, vascular surgery, hematology, cardiac surgery, and clinical pharmacy. To date, there are officially 89 institutions who are a part of the PERT consortium (17).
- PERT are useful in the decision making of not only submassive or massive PE, but also low risk PE with complex comorbidities. Though there has not been a formal randomized controlled trial to evaluate the PERT approach for survival, complications, and cost effectiveness, there have been several retrospective and prospective studies that have addressed these questions. Overall, PERT allows earlier access to advanced therapies, a streamlined approach to facilitate multidisciplinary communication, and an ability to quickly mobilize resources. There has been a proportional increase in the utilization of advanced therapies based on descriptive studies (from 9-19% in one study) without a significant increase in bleeding complications or mortality (20).
- One interesting development is the incorporation of AI. AI is at the forefront of cardiovascular care now, and this includes PE management. There are AI powered PE care coordination software (Viz AI for example), which have been useful in resource limited settings. Alerts are provided to the team with relevant clinical information and even automated imaging analysis (21).
- Though bringing together multiple perspectives can be helpful, it is also resource intensive that requires infrastructure. Future research is needed also in the costs associated with developing and maintaining a team. Outpatient follow up is additionally an important area for growth, specifically with post procedural care, medication adherence, and age appropriate cancer screening (2).
References
- Nguyen, P. C., Stevens, H., Peter, K., & McFadyen, J. D. (2021). Submassive pulmonary embolism: current perspectives and future directions. Journal of Clinical Medicine, 10(15), 3383.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8347177/#:~:text=What%20Is%20the%20Definition%20of,%2C%20and%20standard%2Drisk%20PE.
- Jaff, M. R., McMurtry, M. S., Archer, S. L., Cushman, M., Goldenberg, N., Goldhaber, S. Z., … & Zierler, B. K. (2011). Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation, 123(16), 1788-1830. https://www.ahajournals.org/doi/10.1161/cir.0b013e318214914f#d1e2825
- Konstantinides, S. V., Meyer, G., Becattini, C., Bueno, H., Geersing, G. J., Harjola, V. P., … & Zamorano, J. L. (2020). 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). European heart journal, 41(4), 543-603. https://academic.oup.com/eurheartj/article/41/4/543/5556136
- Chodakowski, J. D., & Courtney, D. M. (2018). Pulmonary embolism critical care update: prognosis, treatment, and research gaps. Current opinion in critical care, 24(6), 540-546. https://journals.lww.com/co-criticalcare/Abstract/2018/12000/Pulmonary_embolism_critical_care_update_.18.aspx
- Ronny, C., Pablo, L., Victor, N., & Brooks, M. (2012). Echocardiographic findings in pulmonary embolism: An important guide for the management of the patient. World Journal of Cardiovascular Diseases, 2012. DOI:10.4236/wjcd.2012.23027
- Mattia Arrigo, Lars Christian Huber, Pulmonary Embolism and Heart Failure: A Reappraisal, Cardiac Failure Review 2021;7:e03. https://doi.org/10.15420/cfr.2020.26
- Piazza, G. (2020). Advanced management of intermediate-and high-risk pulmonary embolism: JACC focus seminar. Journal of the American College of Cardiology, 76(18), 2117-2127. https://www.jacc.org/doi/abs/10.1016/j.jacc.2020.05.028
- Meyer G, et al. “Fibrinolysis for patients with intermediate-risk pulmonary embolism”. The New England Journal of Medicine. 2014. 370(15):1402-1411. https://www.nejm.org/doi/full/10.1056/NEJMoa1302097
- Sharifi M, et al. “Moderate pulmonary embolism treated with thrombolysis”. The American Journal of Cardiology. 2013. 111(2):273-277. https://www.sciencedirect.com/science/article/abs/pii/S0002914912022059
- Pei, D. T., Liu, J., Yaqoob, M., Ahmad, W., Bandeali, S. S., Hamzeh, I. R., … & Alam, M. (2019). Meta-analysis of catheter directed ultrasound-assisted thrombolysis in pulmonary embolism. The American journal of cardiology, 124(9), 1470-1477. https://www.sciencedirect.com/science/article/abs/pii/S0002914919308707
- Kucher, N., Boekstegers, P., Müller, O. J., Kupatt, C., Beyer-Westendorf, J., Heitzer, T., … & Baumgartner, I. (2014). Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation, 129(4), 479-486. https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.113.005544
- Piazza, G., Hohlfelder, B., Jaff, M. R., Ouriel, K., Engelhardt, T. C., Sterling, K. M., … & SEATTLE II Investigators. (2015). A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. Cardiovascular Interventions, 8(10), 1382-1392. https://www.jacc.org/doi/abs/10.1016/j.jcin.2015.04.020
- Tapson, V. F., Sterling, K., Jones, N., Elder, M., Tripathy, U., Brower, J., … & Goldhaber, S. Z. (2018). A randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate-risk pulmonary embolism: the OPTALYSE PE trial. JACC: Cardiovascular Interventions, 11(14), 1401-1410. https://www.jacc.org/doi/abs/10.1016/j.jcin.2018.04.008
- Avgerinos, E. D., Jaber, W., Lacomis, J., Markel, K., McDaniel, M., Rivera-Lebron, B. N., … & Chaer, R. (2021). Randomized trial comparing standard versus ultrasound-assisted thrombolysis for submassive pulmonary embolism: the SUNSET sPE trial. Cardiovascular Interventions, 14(12), 1364-1373. https://www.jacc.org/doi/abs/10.1016/j.jcin.2021.04.049
- Bishay, V. L., Adenikinju, O., & Todd, R. (2021). FlowTriever Retrieval System for the treatment of pulmonary embolism: overview of its safety and efficacy. Expert Review of Medical Devices, 18(11), 1039-1048. https://www.tandfonline.com/doi/abs/10.1080/17434440.2021.1982379
- Piazza, G. (2020). Advanced management of intermediate-and high-risk pulmonary embolism: JACC focus seminar. Journal of the American College of Cardiology, 76(18), 2117-2127. https://www.jacc.org/doi/abs/10.1016/j.jacc.2020.05.028
- Rosovsky R, Zhao K, Sista A, Rivera-Lebron B, Kabrhel C. Pulmonary embolism response teams: Purpose, evidence for efficacy, and future research directions. Res Pract Thromb Haemost. 2019;3(3):315-330. Published 2019 Jun 9. doi:10.1002/rth2.12216. https://www.sciencedirect.com/science/article/pii/S2475037922016181
- Kahn SR, Akaberi A, Granton JT, Anderson DR, Wells PS, Rodger MA, et al. Quality of life, dyspnea, and functional exercise capacity following a first episode of pulmonary embolism: results of the ELOPE cohort study. Am J Med. 2017;130:990 e9–e21. [PubMed]
- Stevinson BG, Hernandez‐Nino J, Rose G, Kline JA. Echocardiographic and functional cardiopulmonary problems 6 months after first‐time pulmonary embolism in previously healthy patients. Eur Heart J. 2007;28:2517–24. https://academic.oup.com/eurheartj/article/28/20/2517/415870
- Rosovsky R, Chang Y, Rosenfield K, Channick R, Jaff MR, Weinberg I, et al. Changes in treatment and outcomes after creation of a pulmonary embolism response team (PERT), a 10‐year analysis. J Thromb Thrombolysis. 2018;47:31-40. [PubMed] )






 Visit Podcast Website
Visit Podcast Website RSS Podcast Feed
RSS Podcast Feed Subscribe
Subscribe
 Add to MyCast
Add to MyCast